Research Programs in the Laboratory
I. Essential roles of synaptic transmission in brain functions
II. Understanding the molecular basis of synaptic function
III. New properties of synapses revealed by imaging approaches
III-1. Synapses are continuously built and eliminated during development
When new synapses are formed, both presynaptic release machinery and postsynaptic receptor should be recruited. Molecular assembly and structural change of single synapses were visualized by using synaptophysin-CFP as a presynaptic marker and PSD-95-YFP as a postsynaptic marker. This imaging analysis revealed the rapid formation of synaptic molecular specialization in the time scale of 30 min to several hours. Global analysis of synapse formation indicated a gradual increase of synapse density, which led to the contention that the formation and maturation of single synapses are also slow and progressive. However, the visualization of individual synapses clearly illustrated the rapid establishment of single synapses.
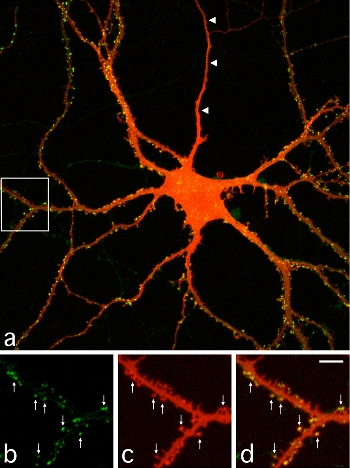
(Legend of Figure 1)
A cultured hippocampal neuron expressing PSD-95 tagged with GFP. The image shows the intracellular distribution of PSD-95 (Green) and neuronal morphology detected by DiI (Red). Images b-d (magnified images of the white box area in a) show spines containing PSD-95 clusters (arrows).
Okabe, S., Kim, H., Miwa, A., Kuriu, T., and H. Okado. Continual remodeling of postsynaptic density and its regulation by synaptic activity. Nature Neuroscience, 2, 804-811, 1999.
Okabe, S., Miwa, A., and H. Okado. Spine formation and correlated assembly of presynaptic and postsynaptic molecules. Journal of Neuroscience, 21, 6105-6114, 2001.
Ebihara, T., Kawabata, I., Usui, S., Sobue, K., and S. Okabe. Synchronized formation and remodeling of postsynaptic densities: long-term visualization of hippocampal neurons expressing postsynaptic density proteins tagged with GFP. Journal of Neuroscience, 23, 2170-2181, 2003.
III-2. Postsynaptic scaffolding molecules are dynamic and densely packed
By using a technique of fluorescence recovery after photobleaching (FRAP), turnover rates of PSD scaffolding molecules were estimated at the level of single synapses. Time constants of turnover were in a range of several minutes to several tens of minutes, indicating a faster assembly-disassembly rate of scaffolding molecules compared with the lifetime of the PSDs themselves. These data suggest that PSD scaffolding molecules are densely packed and have multiple interactions, but in the process of frequent exchange with the soluble cytoplasmic pool, which enables continual changes in the size and structure of the PSDs.
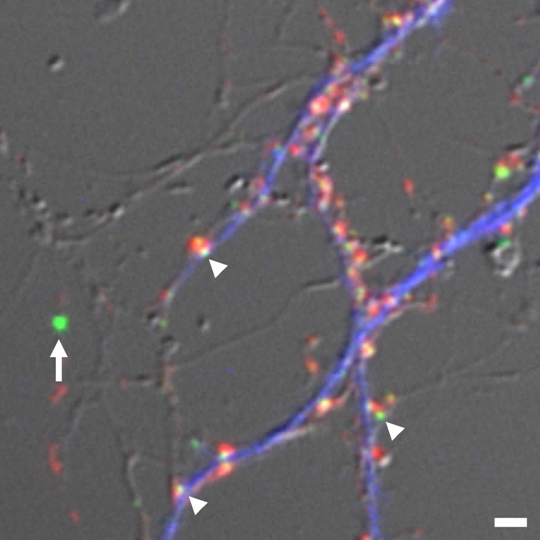
(Legend of Figure 2)
Estimation of absolute numbers of GFP-tagged PSD scaffolding molecules. We determined the relative fluorescence ratio of fluorescent microspheres (arrow), calibrated against single GFP molecules, and individual PSD clusters (arrowheads). We estimated absolute numbers of GFP-tagged PSD scaffolding molecules in single synapses. Presynaptic boutons (red) and dendritic shafts (blue) were also identified by synaptophysin staining and MAP2 staining, respectively.
Sugiyama, Y., Kawabata, I., Sobue, K., and S. Okabe Determination of absolute protein numbers in single synapses by a GFP-based calibration technique. Nature Methods 2, 677-684, 2005.
Okabe, S., Urushido, T., Konno, D., Okado, H., and K. Sobue. Rapid redistribution of the postsynaptic density protein PSD-Zip45 (Homer 1c) and its differential regulation by NMDA receptors and calcium channels. Journal of Neuroscience, 21,9561-9571, 2001.
Kuriu, T., Inoue, A., Bito, H., Sobue, K., and S. Okabe Differential control of postsynaptic density scaffolds via actin-dependent and independent mechanisms. Journal of Neuroscience 26, 7693-7706, 2006.
III-3. Astrocytic contacts promote synaptogenesis
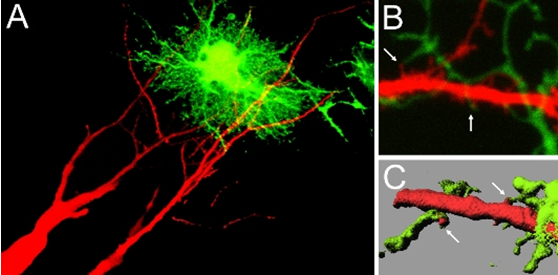
(Legend of Figure 3)
Contact between dendritic spines and astrocytic processes. In A, hippocampal pyramidal neurons were filled with a red fluorescent dye, and astrocytes were infected with recombinant adenoviruses for GFP expression. Contact sites can be identified. In B, two-photon imaging revealed individual contact sites between spines (red) and astrocytic processes (greed). C shows an image after surface rendering, which facilitates the visualization of contact sites between two cell types (arrows).
Nishida, H. and S. Okabe Direct astrocytic contacts regulate local maturation of dendritic spines. Journal of Neuroscience 27, 331-340, 2007.
III-4. Diversity in synapse formation
The second example of unique strategies taken by specific neurons in synapse formation was found in the cerebellum. Detailed analyses of postnatal synapse development between cerebellar Purkinje cells and granule cell axons (parallel fibers) led to the discovery of small axonal protrusions at the contact sites with Purkinje cell dendrites. These axonal protrusions of granule cells wrap dendritic spines of Purkinje cells and facilitate synapse maturation. The formation of axonal protrusion was mediated by a signaling pathway triggered by a soluble factor cbln1. This finding revealed sequential interplay between dendrites and axons plays an essential role in synapse maturation.
Even in the case of excitatory synapses in pyramidal neurons, which have been studied extensively, new principles and new regulatory molecules could be discovered. Microtubule-associated protein DCLK1 shows the robust activity of facilitating microtubule assembly and stabilization. Interestingly, DCLK1 is preferentially localized at the tips of growing dendrites. This unique localization of DCLK1 helps the facilitation of microtubule assembly at the tips of dendrites. On the other hand, DCLK1 shows suppressive roles in spine growth, assembly of PSD molecules, and postsynaptic receptor functions. Namely, DCLK1 controls dendritic growth positively and synapse maturation negatively at the tips of growing dendrites. The dual roles of DCLK1 may be critical in local control of dendritic development.
After the discovery of a new mechanism of synapse maturation based on presynaptically released cbln1, we initiated the screening of other molecular candidates that are released from the axons and regulate synapse formation. We identified BMP4 as a negative regulator of synapse formation in the hippocampal excitatory synapses. BMP4 acts on BMP receptors on the surface of the axonal membrane and activates the signaling cascade that facilitates the deconstruction of the presynaptic structure. BMP4 keeps the synapse density within the physiological range by removing excess and unnecessary synapses in the hippocampal neural circuits.
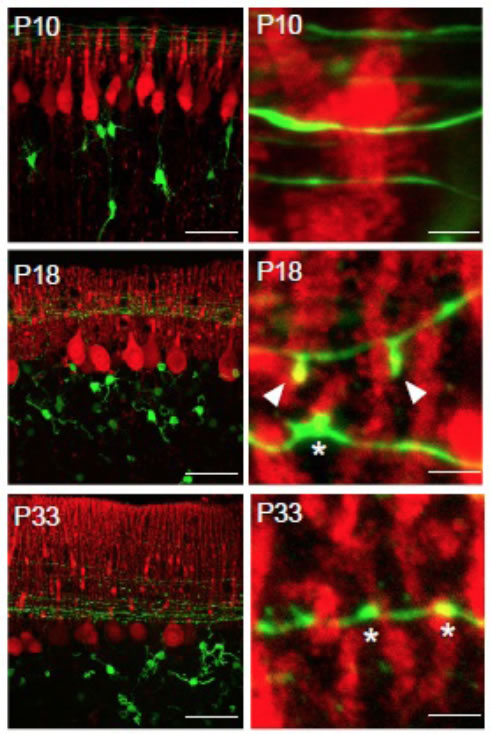
(Legend of Figure 4)
Developmental changes in the morphology of synapses between parallel fibers (GFP signal, green) and Purkinje cell dendrites (anti-calbindin staining, red). At postnatal day 18, unique protrusive structures (arrowheads) can be identified, which contact with Purkinje cell spines.
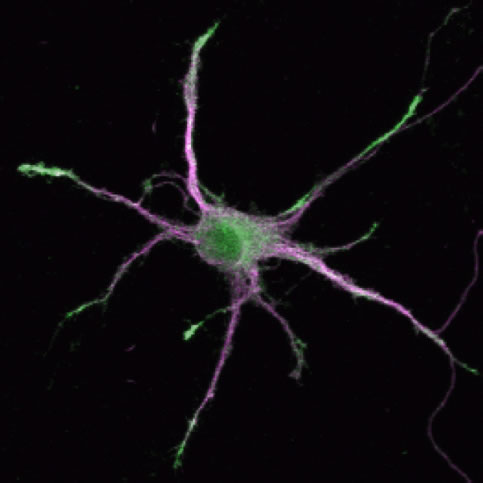
(Legend of Figure 5)
DCLK1 is localized at the growing tips of dendrites and facilitate process growth. On the other hand, DCLK1 suppresses the maturation of synapses locally and keep the dynamics of distal dendrites. Green; anti-DCLK staining, Magenta; distribution of a dendritic marker MAP2.
Kawabata, I., Kashiwagi, Y., Obashi, K., Ohkura, M., Nakai, J., Wynshaw-Boris, A., Yanagawa, Y., and S. Okabe LIS1-dependent retrograde translocation of excitatory synapses in developing interneuron dendrites. Nature Communications 3, 722, 2012.74.
Ito-Ishida, A., Miyazaki, T., Miura, E., Matsuda, K., Watanabe, M., Yuzaki, M and S. Okabe Presynaptically released Cbln1 induces dynamic axonal structural changes by interacting with GluD2 during cerebellar synapse formation. Neuron 76, 549-564, 2012.
Shin, E., Kashiwagi, Y., Kuriu, T., Iwasaki, H., Tanaka, T., Koizumi, H., Gleeson, J. G. and S. Okabe Doublecortin-like kinase enhances dendritic remodeling and negatively regulates synapse maturation. Nature Communications 4, 1440, 2013.
Higashi T, Tanaka S, Iida T, and S. Okabe Synapse elimination triggered by BMP4 exocytosis and presynaptic BMP receptor activation. Cell Reports 22, 919-929, 2018.
IV. Application of synapse imaging in studies of mental disorders
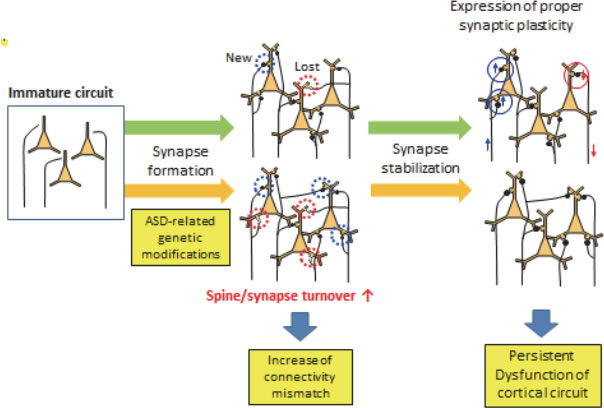
(Legend of Figure 6)
Proposed neural circuit alterations present in mouse models of ASDs. ASD-related genetic modifications will alter the speed of synapse turnover, which may increase the mismatches between pre- and postsynaptic components. These mismatches affect the proper functions of the cortical neural circuits, which may be fixed after the suppression of synapse dynamics in the adult stage.
Isshiki, M., Tanaka, S., Kuriu, T., Tabuchi, K., Takumi, T. and S. Okabe Enhanced synapse remodelling as a common phenotype in mouse models of autism. Nature Communications 5, 4742, 2014.
V. New technologies in synapse imaging
The application of fluorescence correlation techniques can report molecular diffusion within the spine cytoplasm. We found the intra-spine suppression of diffusion for the molecules with their sizes larger than 100 kDa. The diffusional barrier is actin-filament dependent and can be disassembled by induction of spine structural plasticity only for 5 minutes after plasticity-inducing stimulation. The highly temporally restricted window of molecular mobilization is a new concept, which may contribute to define a distinct stage in synaptic plasticity.
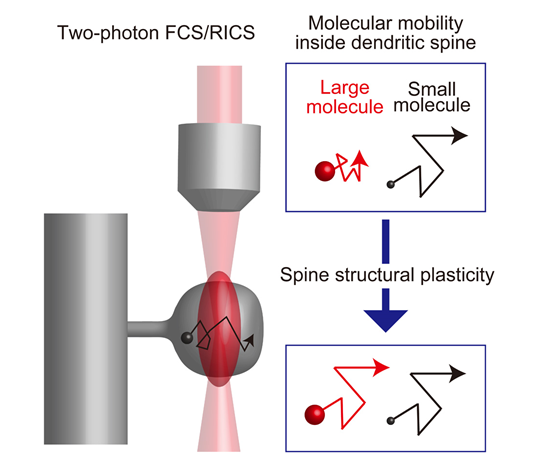
(Legend of Figure 7)
Molecular mobility can be directly measured by fluorescence correlation spectroscopy. After the induction of synaptic plasticity, the suppression of movement in molecules larger than 100 kDa is specifically released. Many important signaling molecules within spines are of high molecular weight and may be affected by this release mechanism.
Kashiwagi, Y., Higashi, T., Obashi, K., Sato, Y., Komiyama, N., Grant, S. G. N. and S. Okabe Computational geometry analysis of dendritic spines by structured illumination microscopy. Nature Communications 10, 1285, 2019.
Obashi, K., Matsuda, A., Inoue, Y., and S. Okabe Precise temporal regulation of molecular diffusion within dendritic spines by actin polymers during structural plasticity. Cell Reports 27, 1503-1515, 2019.
VI. Future perspectives
The cerebral cortex, responsible for the higher cognitive functions, is the brain structure highly developed in mammals, especially in the primates. The underlying architecture of the cerebral cortex is preserved across the mammalian species and is the parallel repetition of the columnar structural units containing both excitatory and inhibitory neurons. The well-known examples of the columnar units are the ocular dominance column and the orientation column in the primate visual cortex. It is also demonstrated that smaller columnar structures, called mini-columns, are present in the cerebral cortex, which are formed along the radial axis of the excitatory neuron migration in the developmental stage. The relationship between the large functional columns and the small mini-columns is not yet clear. Furthermore, the projection patterns of neurons in the mini-columns to the other cortical neurons and the connectivity of excitatory and inhibitory neurons within the mini-columns need to be clarified. Using the mini-column as a model of the minimal cortical unit, the design principles of the synaptic connectivity may be investigated systematically.
Our imaging studies in the culture system demonstrated characteristic changes in the spine nano-structure after induction of spine plasticity. However, it is not yet clear if the spine nano-structure shows any modifications in the process of learning and memory. Activity-dependent gene expression in the learning paradigms, such as fear conditioning, can be used to label and manipulate a specific subset of neurons (engram cells), which are involved in the formation of memory-related neural circuits. It is crucial to compare the spine nano-structure in the synapses between engram cells and evaluate the detected nano-scale changes in comparison with the structural changes induced in vitro. Furthermore, we should test if the nano-scale changes in spine synapses are responsible for the functional alterations of neural circuits underlying learning.

